Current Research Projects
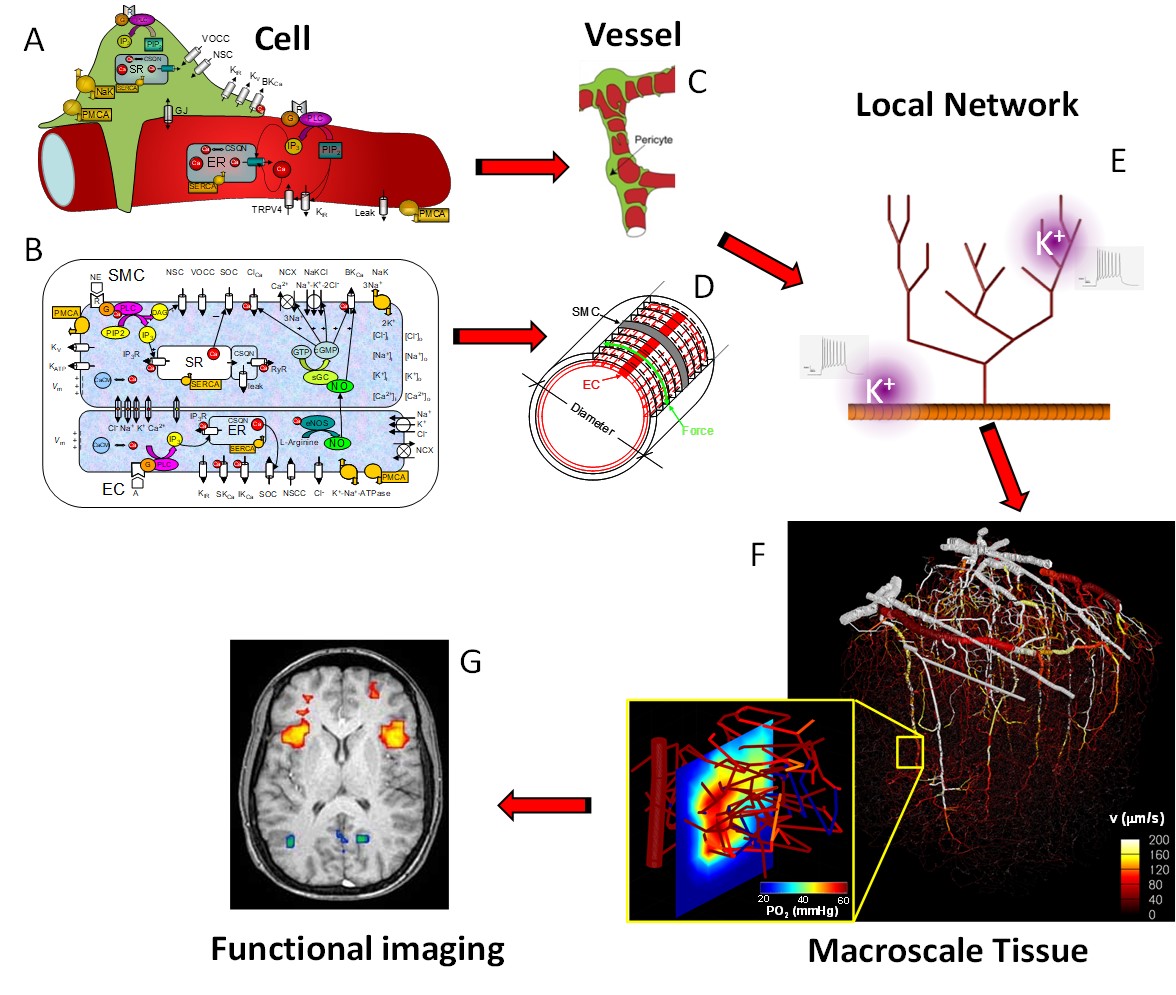
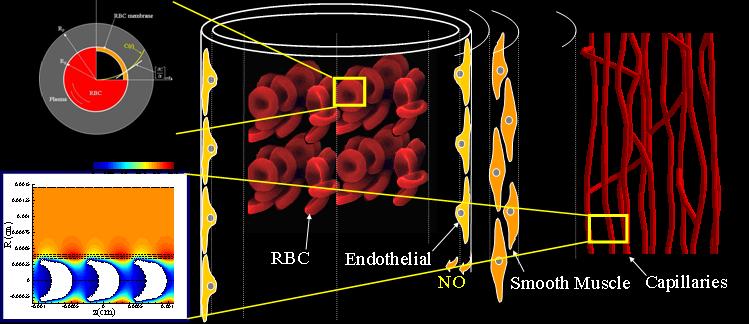
Neurovascular coupling: from cell signaling to fMRI: Over the last decade we have focused on the development of a multiscale mathematical modeling approach to investigate the regulation of blood flow and tissue The objective is to link macroscopic physiological function at the tissue/organ level to the underlying molecular/subcellular mechanisms. At the tissue level we have presented the detailed models of O2 transport from three-dimensional vascular networks . At the vessel level we have examined vasoactive signaling mechanisms focusing mostly on Endothelial Derived Hyperpolarization (EDH) and Nitric Oxide-dependent vasoreactivity. At the cell level we have presented mathematical models of Ca2+ dynamics and electrophysiology in Endothelial (EC) and Smooth Muscle Cells (SMC).
Tsoukias, M., D. Goldman, A. Vadapalli, R. N. Pittman and A. S. Popel. A computational model of oxygen delivery by hemoglobin-based oxygen carriers in three dimensional microvascular networks. Journal of Theoretical Biology 248(4):657-74, 2007.
Kapela A, Tsoukias N.M. Multi-Scale FEM Modeling of Vascular Tone: From Membrane Currents to Vessel Mechanics. IEEE Trans Biomed Eng. 58(12):3456-59, 2011.
Nitric Oxide Biotransport: Despite significant prior work on the vasoreactive role of NO, the factors that regulate NO bioavailability in the vasculature are still under For example it is not known how NO escapes scavenging by the erythrocytic hemoglobin and what NO levels can be maintained in the vascular wall to induce vasodilation (reported/predicted NO concentrations range from pico to micro molar). Through mathematical modeling we have been able to quantify some of these interactions and to make physiological relevant predictions. We have shown for example, that NO activity can be regulated by the frequency of the underlying Ca2+ events. We have tested experimental approaches for assessing NO in biological tissues and highlighted the limitations of existing fluorescent markers and we have elucidated the kinetics of NO preservation through the formation of Nitrosothiols. Our combined theoretical and experimental methodology has provided insights for the fate of NO in the vasculature.
Tsoukias, M., M. Kavdia and A.S. Popel. A theoretical model of nitric oxide transport in arterioles: frequency vs amplitude dependent control of cGMP formation. American Journal of Physiology 286(3):H1043-56, 2004.
Madrasi, T. Gadkari, M. S. Joshi and N.M. Tsoukias. Glutathiyl Radical as an Intermediate in the Glutathione Nitrosation. Free Radic Biol Med; 53(10):1968-76, 2012.
Namin , S. Nofallah, M. Joshi and N.M. Tsoukias. Kinetic analysis of Diaminofluorescein activation by NO: Toward calibration of a NO-sensitive fluorescence probe. Nitric Oxide, 28:39-46, 2013.
Gadkari V., N. Cortes, N M. Tsoukias, and M. S. Joshi. Agmatine Induced NO Dependent Rat Mesenteric Artery Relaxation and its Impairment in Salt-Sensitive Hypertension. Nitric Oxide, 35C:65- 71, 2013.
Exhaled NO: We investigate the dynamics of nitric oxide in the exhaled breath and its potential as a diagnostic test of lung. In the late 90’s we observed that the concentration of NO in the exhaled breath depends on the expiratory flow rate. This discovery prompted the American Thoracic Society to issue recommendations for the standardization of exhaled NO monitoring. Our theoretical models explained this phenomenon and provided a method to analyze and to partition the exhaled NO levels into an alveolar and airway origin. Our proposed method of analysis has been widely used in the literature and in clinical studies over the last fifteen years.
Tsoukias, M., Z. Tannous, A. F. Wilson and S. C. George. Single Exhalation Profiles of NO and CO2 in Humans: Effect of Dynamically Changing Flow Rate. Journal of Applied Physiology 85(2):642-652, 1998.
Tsoukias, M. and S. C. George. A Two-Compartment Model of Pulmonary Nitric Oxide Exchange Dynamics. Journal of Applied Physiology 85(2):653-666, 1998.
Tsoukias, M. and S. C. George. A Single Breath technique with variable flow rate to characterize Nitric Oxide Exchange Dynamics in the Lungs. Journal of Applied Physiology 91(1):477-487, 2001.
George C. and N.M. Tsoukias. An apparatus and method for the estimation of flow independent parameters which characterize the relevant features of Nitric Oxide production and exchange in the human lungs. (WO 01/82782; US 6,866,637).
